One of the more useful ways San Diego contributes to society is with world-leading biotech. Even though I’m really a computer specialist, I spent about 15 years working in molecular biology labs there. One does not hang around computational molecular biophysicists that long without learning some basics.
One of the fun benchmarks I used to do is assemble icosohedral hepatitis capsids into the full virus where every atom is properly placed. It’s interesting to see a machine where you know the location of every atom. I was thinking of assembling a COVID19 virus' helical capsid but I don’t think there are any solved structures of the proteins this virus is comprised of.
Searching PDB the only thing that seems to exist at this time is a cryo-electromicroscopy structure of the spike glycoprotein trimer. This is the structure, i.e. the placement of each atom, of the spiky protrusions sticking out of COVID19.
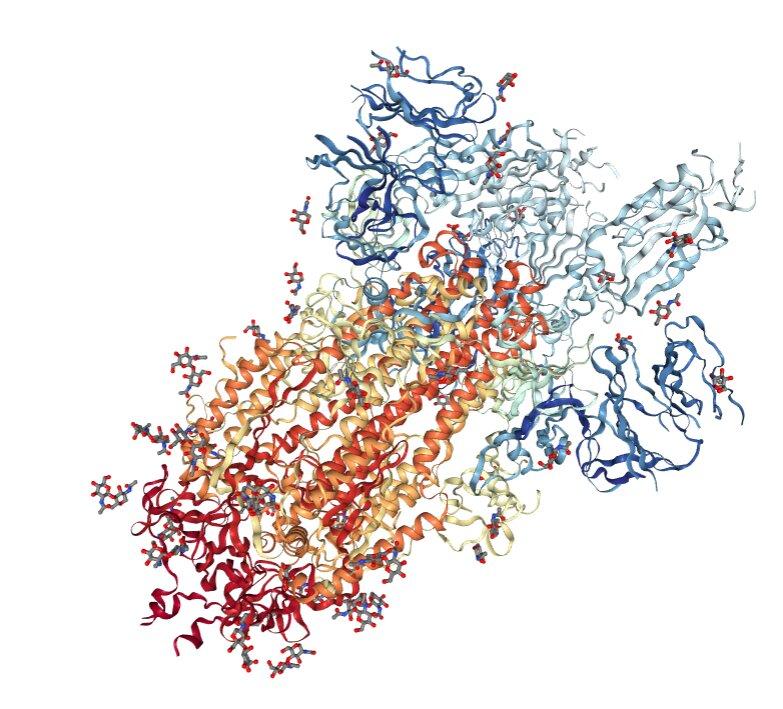
You don’t have to be an expert to see that this is hasty work. This is a trimer, three identical protiens in a trigonal symmetry. But one of them clearly isn’t like the others, is it? They provide excuses in the paper.
While looking at that one thing caught my eye, a video titled Fighting Coronavirus With Soap. One thing that has always intrigued me is soap. How does it work? I mean how does it really work? The video is only a couple of minutes and graphically quite well done.
Watching the video, I was a little uneasy about the message. Let’s learn more about soap’s molecular physics and think this through.
This is how the video is representing a soap molecule.
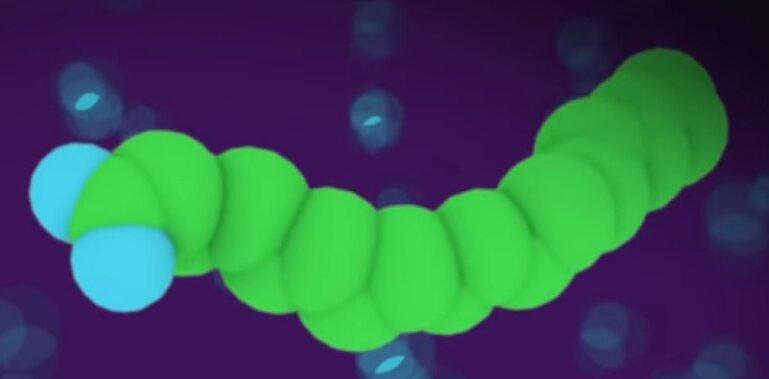
Here is a more technical representation of stearic acid which is found in many common soaps.
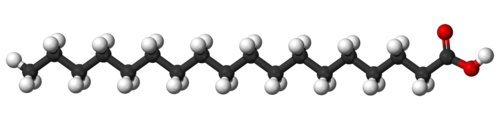
The white spheres are hydrogen, the black spheres are carbon, and the red are oxygen. The carbon chain is constructed like a fat. This is why soap has some similarities to oily stuff; for example soap can be used to lubricate things.
So let’s say that you have dispersed a bunch of these molecules into water and the important property of the lipid tail is that it hates getting wet. Of course at molecular levels the idea of "wet" isn’t exactly a thing, but that’s fine. The tails of soap molecules are what’s called "hydrophobic" — if there’s a way for them to avoid water and bond to something else, they will. The heads are "hydrophilic", they love water!
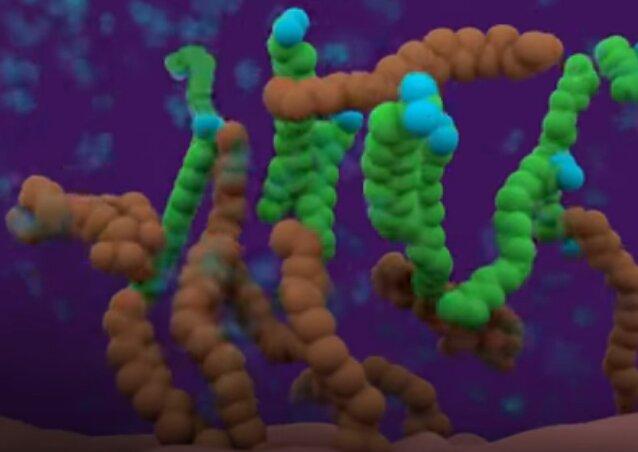
Now let’s say you have some surface that is covered in oily lipids. The video illustrates such a situation like this.

The brown strands are just like the soap’s tails. The idea — and indeed molecular fact — is that when the soap tails get near these oil residue tails it is more "comfortable" (electrochemically) backing its soap tail into the lipids to get away from the water.
If enough of this activity happens, the soap and lipid cluster can form a little ball called a micelle.
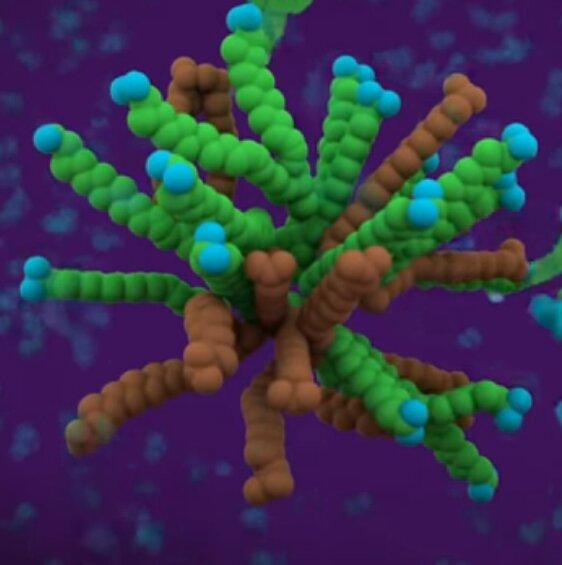
This ball is the most comfortable configuration (minimal energy state) for the soap and any lipids it gets near to avoid the water. Once the lipids are bound up in a little ball whose outside surface is happy to swim around in water, it turns out that it is easy to rinse the whole assembly away. This is why if you put soapy water in greasy cookwear, the stubborn residue can finally be rinsed clean. The oils of your food are generally the same stuff for the purposes of this topic.
We’ve seen soap form into little balls called micelles. There are other configurations such molecules can take. This Wikipedia image is called "phospholipids_aqueous_solution_structures".
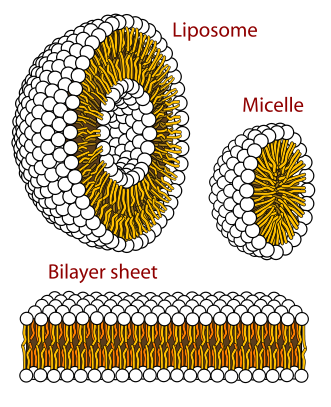
There’s the micelle as described. Then there are the bilayer types. These form as shown when two sheets are arranged tail to tail, again neatly avoiding the tail getting "wet". Next to think about are liposomes. Liposomes are small little spheres of this molecular configuration. Researchers are trying to figure out how to pack them with therapeutic agents in order to sneak drug metabolites into a patient in a way that otherwise would dissolve in water. They hope it can be a clever way to sneak something into some place it doesn’t usually go.
This is exactly how a virus works! A virus envelope (the round sphere you see in all the images of the covid 19) is the same topological bi-layer organization of lipids as a liposome — it is just somewhat bigger.
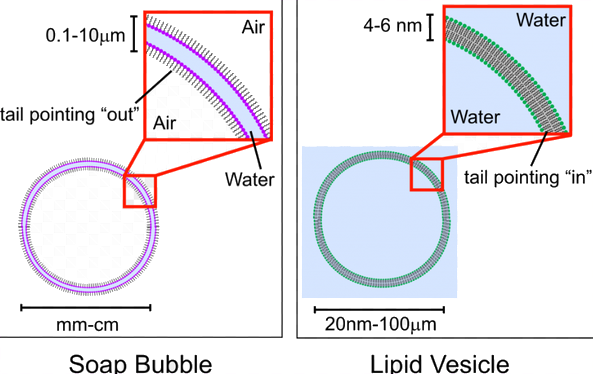
It is also interesting to note that soap bubbles are formed by the same mechanics, just sort of in reverse. This diagram illustrates it well.
The video shows the virus' envelope bilayer sheet like this.
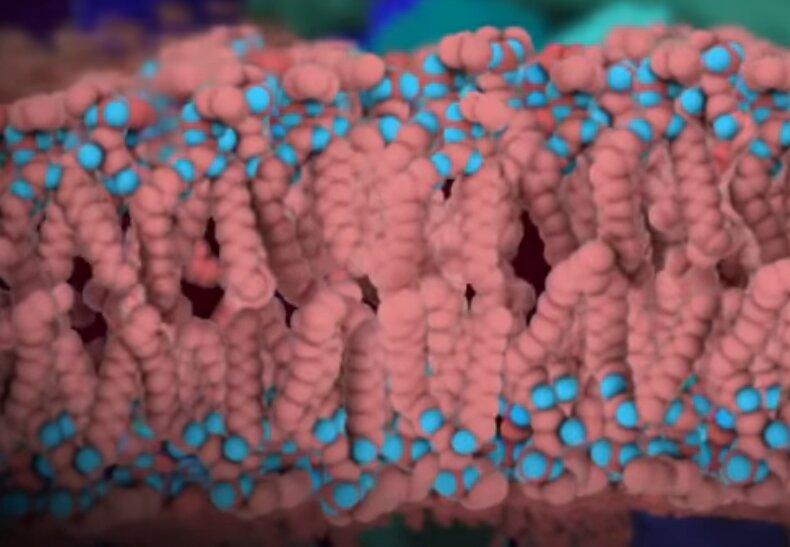
What exactly is that phospholipid layer of virus envelope made of? Wikipedia says "The envelopes are typically derived from portions of the host cell membranes (phospholipids and proteins)…" Did you catch that? Imagine two kids playing with a limited collection of Legos — one takes a break and returns to find their construction half-destroyed and the other’s now greatly expanded. Viruses steal your cell membranes to make their own membranes! At an atomic level virus skin is the exact same stuff as your skin! Well, the "skin" of your skin — and any other handy tissue — meaning the cell membranes. Viruses are perfectly effective even when they destroy their hosts' cellular machinery. And when cells fail and disintegrate, what is conveniently lying around in abundance for an ambitious virus to use? Your cells' membrane lipids!
And now we get to the problem. If soap can polarize bilayer lipids (or proteins) of virus envelopes, well, I’ve got some bad news for you about what it will be doing to the membranes of your skin cells. Yes, viruses exposed to soap in high enough concentrations for long enough periods of time may start to become unstable for effectively the same reasons that sandpaper, battery acid, and open flames all can destabilize viral envelopes (i.e. the wrong energy in the wrong place) but I do not think this is what the essence of soap hygiene is all about.
I can accept that the action of soap can be a little rough. You certainly don’t like to eat it and it can irritate other sensitive areas like your eyes. An interesting property of skin cells (which are called keratinocytes) is that they usually are completely dead on the very outside of your body. You are covered in dead skin which protects you by absorbing damage that chemical assaults like soap entail.
Now we have some important facts that can help us understand the best way to use soap.
-
Soap does nothing more than polarize lipids (making oily things easy to rinse away with water).
-
Although your skin and virus envelopes are somewhat resilient to soap, both can be damaged by soap’s destabilizing effects on your tissues' lipid bilayer in the right circumstances.
-
Your skin generally has a shell of dead skin cells that can absorb some environmental damage, including that of soap.
So what could this mean for washing and dangerous pathogen mitigation? Here are some things to consider.
-
If you have oil on your skin — and your sebaceous glands tell me that you do — it may be easy to trap viruses in that oil.
-
There are no sebaceous glands on the palms, but if you touch something icky (or someone), or your own body, you could pick up oil that could be carrying viruses.
-
Touching things other people touched could also transfer such oil. Think about what you’re touching and what kind of oils/lipids might be there. If police investigators could get fingerprints from it, I would consider it possible to transfer oils to your skin.
-
Washing this oil off seems reasonable and soap will make it happen.
-
Poop is produced by the system where waste lipids are separated from waste water. If you have any reason to suspect there is poop on you, soap is a great idea.
-
Putting your hands in/near your mouth or eating food that you’re holding is especially problematic and needs your hands to be free of dangerous pathogens.
-
If you’re going to perform surgery or something like that, maybe scrubbing down until you damage your own skin is reasonable.
-
If you are a surgeon, and wherever anyone is in a serious medical situation, wear gloves.
-
Not only are the gloves important, but being mindful of what you touch while wearing them.
That’s all pretty normal sounding, right? You won’t be surprised to learn I have some more controversial notions. Of course I do!
-
Destroying your skin with harsh surfactants is harsh and perhaps not ideal.
-
Obsessive hand washing is obsessive. Think it through!
-
Destroying your skin’s natural oils completely can not be ideal. There is a balance!
-
For the same reason that putting skin oil in your mouth can be a bad idea when trying to avoid infection, that oil may also be helpfully trapping and immobilizing pathogens.
-
It’s possible that virus envelopes are more delicate than your keratinocyte (skin cell) membranes because the viruses mostly replicate in — and are therefore made up of — the respiratory system where soap might be more destructive. If that is plausible then simply drying or heating your hands would also be as helpful as soap. Basically doing anything that your outside tolerates better than your inside.
-
And here’s the hard one to sell — your body’s immune system builds upon measured exposure to bad things. It’s all about concentrations. If you are too aggressive about flensing your skin’s microbiome, you could damage your body’s ability to form a proper defense.
-
Some chemicals in soap may not be good for you or your skin’s microbiome. Maybe at certain concentrations. Maybe it’s fine when the skin is protected by oils but problematic once they are stripped. Remember, the FDA just banned triclosan from hand soaps in 2016. Before that it was ubiquitous.
The FDA goes on to say:
The FDA is particularly interested in gathering additional data on the long-term safety of daily, repeated exposure to these ingredients by consumers, and on the use of these products by certain populations, including pregnant women and children, for which topical absorption of the active ingredients may be important. Emerging science also suggests that for some antiseptic active ingredients, systemic exposure (full body exposure as shown by detection of antiseptic ingredients in the blood or urine) is higher than previously thought, and that more information is needed about the effects of repeated daily human exposure to some antiseptic active ingredients.
Wash your hands — and other parts — the way that is best for your well-being. With a little biochemistry understanding, you hopefully can make some better guesses about what exactly that way is. Soap is a tool — use it correctly, in the right circumstances, and it will be good to you.
Thanks to the brilliant and kind biophysics professor, Ruben Abagayan for reviewing this topic with me. All errors are mine!